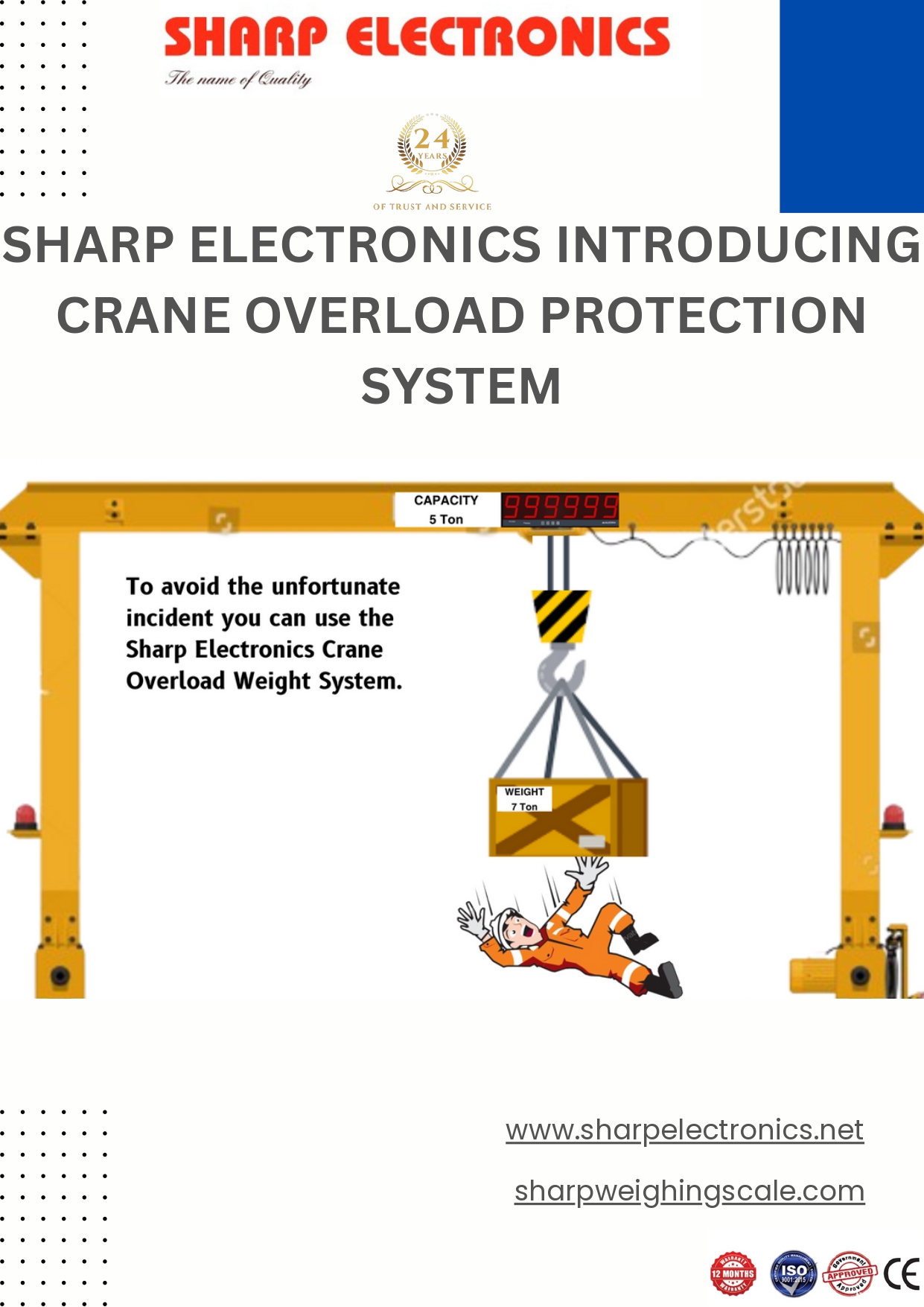
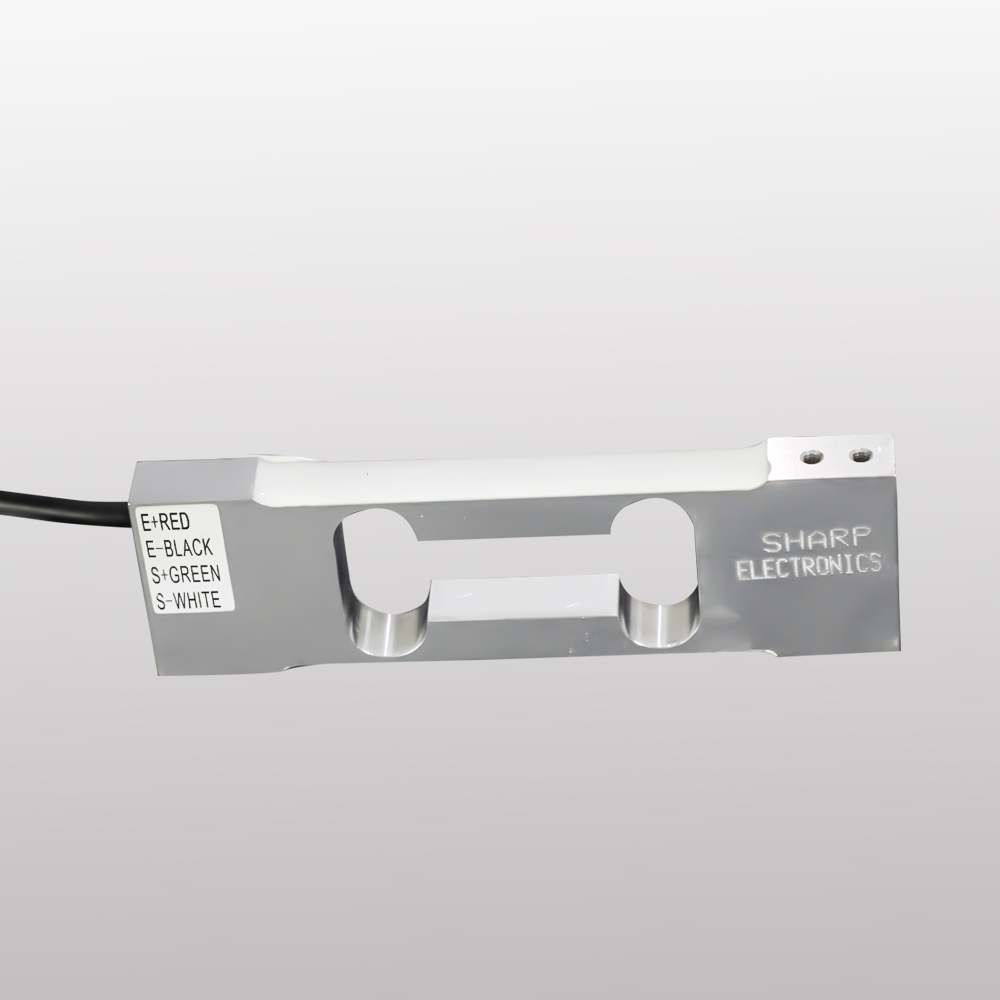
About
Sharp Electronics-Load Cell And Weighing Machine Manufacturers in Pune India
Load Cell Manufacturers And Weighing Machine or Weighing Scale Manufacturers in Pune,Chakan, Bhosari, Talegaon, Ranjangaon, Pirangut, Shirwal, Supa, Baramati, Raigad, Taloja, India|Sharp Electronics
Sharp Electronics, established in 2000, is an ISO 9001:2015 certified organization engaged in the manufacturing and export of high-performance industrial weighing systems. Operating under a partnership firm structure, we specialize in the production or manufacturers of load cell, weighing scale, precision-engineered products, including Tabletop Scales, Platform Scales, Weight Controllers, and customized weighing solutions tailored to industrial and commercial applications.
Our products are extensively deployed in sectors such as retail weighing, agriculture, poultry and fisheries, hardware trading, market yards, and metal industries. With a strong emphasis on accuracy, reliability, and long-term performance, our systems are designed to withstand harsh working environments and deliver consistent output.