by Brookhaven National Laboratory
Overall structure of a transmembrane ZIP zinc transporter protein determined by cryo-electron microscopy (cryo-EM) (left) and a schematic showing some of the functional features (right). A flexible loop (magenta) facing into the cell binds to zinc and folds to block the entry of more zinc when levels of this micronutrient get too high. Credit: Brookhaven National Laboratory
Scientists at the U.S. Department of Energy’s (DOE) Brookhaven National Laboratory have determined the atomic-level structure of a zinc-transporter protein, a molecular machine that regulates levels of this crucial trace metal micronutrient inside cells. As described in a paper just published in Nature Communications, the structure reveals how the cellular membrane protein shifts its shape to move zinc from the environment into a cell, and temporarily blocks this action automatically when zinc levels inside the cell get too high.
“Zinc is important for many biological activities, but too much can be a problem,” said Qun Liu, the Brookhaven Lab biophysicist who led the project. “During evolution, different organisms have evolved in many ways to regulate zinc. But no one has shown that a transporter that controls the uptake of zinc from the environment can regulate its own activity. Our study is the first to show a zinc transporter with such a built-in sensor.”
The research was conducted as part of Brookhaven Lab’s Quantitative Plant Sciences Initiative (QPSI). Using a bacterial version of a zinc transporter that shares essential features with zinc transporters in plants, the scientists gained key insights into how these proteins work.
“This research is part of our effort to understand how micronutrients like zinc are taken up by plants so we can understand how to design plants that are better able to grow on marginal land for the production of bioenergy,” said Brookhaven Lab Biology Department Chair John Shanklin, a co-author on the paper.
The research could also suggest ways to engineer food crops with increased zinc content to improve their nutritional value, the scientists noted.
Cryo-EM plus computation
To solve the protein structure, the Brookhaven team used cryo-electron microscopy (cryo-EM) at the Laboratory for BioMolecular Structure (LBMS). With this technique, scientists can sample many different conformations of a protein instead of a single, crystallized form. That’s important because, in nature, proteins are dynamic, not static; pieces of them move around.
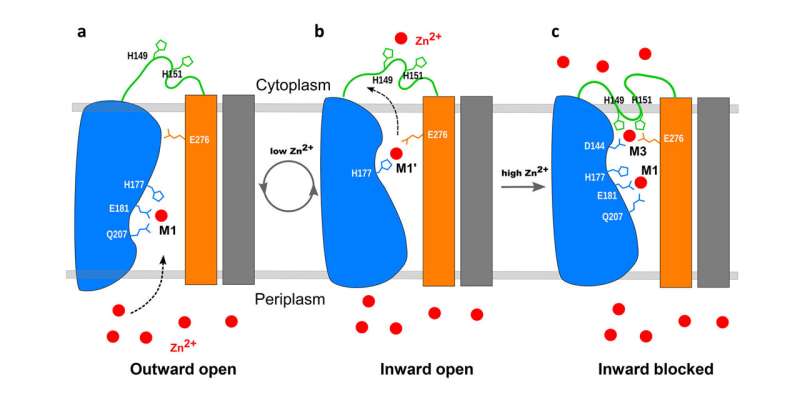
Schematic of the mechanism for the transmembrane ZIP transporter (blue, orange, and green—with gray bars representing part of the other half of the two-molecule dimer). a) When zinc (red) inside the cell (cytoplasm) is low, the part of the protein facing the environment (periplasm) is open so zinc can enter a pore in the protein. b) The blue portion of the protein moves up slightly and tilts to transport zinc into the cell. c) When zinc inside the cell gets too high, it binds to the flexible green loop, which then folds back and binds to sites inside the pore to block the exit of zinc. When zinc levels inside the cell fall, the loop will pop back out to allow zinc to enter again. Credit: Brookhaven National Laboratory
Schematic of the mechanism for the transmembrane ZIP transporter (blue, orange, and green—with gray bars representing part of the other half of the two-molecule dimer). a) When zinc (red) inside the cell (cytoplasm) is low, the part of the protein facing the environment (periplasm) is open so zinc can enter a pore in the protein. b) The blue portion of the protein moves up slightly and tilts to transport zinc into the cell. c) When zinc inside the cell gets too high, it binds to the flexible green loop, which then folds back and binds to sites inside the pore to block the exit of zinc. When zinc levels inside the cell fall, the loop will pop back out to allow zinc to enter again. Credit: Brookhaven National Laboratory
“Cryo-EM does not require proteins to form crystals, so we can actually capture dynamic steps that may not feasible using X-ray crystallography, another technique for studying protein structures,” Liu said. “In essence, with cryo-EM, we can capture more frames of the ‘movie’ to get a structure that is very helpful in understanding a protein’s biological function.”
To sort through the many variations in structure, the scientists need powerful computational tools. These include artificial intelligence approaches that use machine learning, some of which Liu has developed. Using these algorithms, the scientists can semi-automatically select and sort through millions of cryo-EM images to find groups of structures with similarities. The method allows them to achieve the highest feasible resolution, and thus to reveal atomic-scale details of the structure.
For this study, this cryo-EM approach revealed key features of one stage of a ZIP (Zrt-/Irt-like protein) zinc transporter that reveals how it regulates its own zinc-uptake activity depending on how much zinc is already in the cell.
“Our new data made us revise the previous views as to how this protein works,” Liu said.
Tilt to enter, sense to stop
An earlier report based on X-ray crystallography and coevolutional analyses suggested that the transporter may function as a sort of “elevator” to transport zinc. The new research shows how interactions with zinc on either side of the cellular membrane trigger the movement of parts of the protein to bring zinc into the cell—and, crucially, block its entry when the levels inside get too high.
“Our key structure shows that when the zinc level inside the cell rises to a certain level—beyond what’s required to meet the cell’s demands—the excess zinc binds to a loop on the inside of the membrane,” Liu said. “Then, as this flexible loop reorients, it folds back on itself, and binds in a manner that blocks zinc from entering the cell.”
“It’s almost like the plug going into a bathtub drain and blocking it,” Shanklin added.
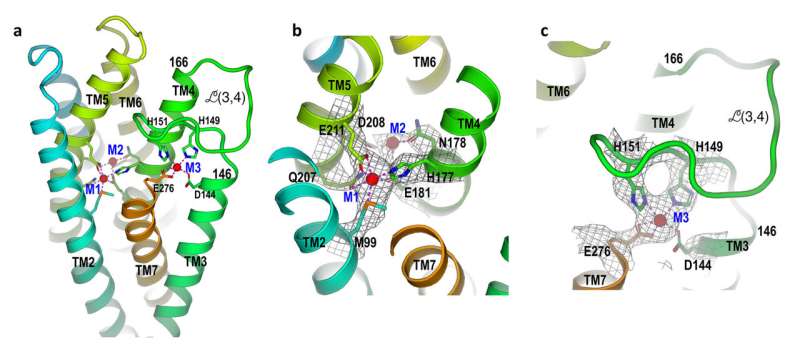
Detailed views of the ZIP protein structure in the zinc-blocking state. Metal binding sites (M1-3) show where zinc binds. The close-up view (c) shows how zinc binding to the M3 location—associated with two histidine residues (H151 and H149) on a flexible green loop portion of the protein—causes the loop to fold and block the exit of zinc from the pore of the protein. Credit: Brookhaven National Laboratory
Detailed views of the ZIP protein structure in the zinc-blocking state. Metal binding sites (M1-3) show where zinc binds. The close-up view (c) shows how zinc binding to the M3 location—associated with two histidine residues (H151 and H149) on a flexible green loop portion of the protein—causes the loop to fold and block the exit of zinc from the pore of the protein. Credit: Brookhaven National Laboratory
The scientists also worked out how other parts of the protein move to allow zinc to enter.
When zinc levels inside the cell are low, zinc falls off the loop portion and the plug pops back out of the transporter. Zinc from the environment can move into the transporter. Inside the transporter, the zinc causes part of the protein machine to move up and tilt, closing the exit to the external environment. Once the zinc moves into the cell, the machine will reset itself to work again.
“Our cryo-EM structure is the first to show how this loop domain of the protein modulates the transporter’s activity by feedback depending on the level of zinc,” Liu said.
It’s also the first structure to show that this zinc transporter is an arrangement of two identical proteins—known as a dimer. “It requires two molecules to do the work,” Liu said.
The scientists think that having two molecules acting in the form of a dimer may be related to its function or stability—which they’ll explore with future computational simulations of how the molecules work together.
“This research could enable new ways of engineering zinc transporters in microbes and plants to optimize their growth in conditions where zinc is too low or too high, potentially on marginal lands for the production of bioenergy and bioproducts,” Liu said.
More information: Changxu Pang et al, Structural mechanism of intracellular autoregulation of zinc uptake in ZIP transporters, Nature Communications (2023). DOI: 10.1038/s41467-023-39010-6
Journal information: Nature Communications
Provided by Brookhaven National Laboratory
More information: Changxu Pang et al, Structural mechanism of intracellular autoregulation of zinc uptake in ZIP transporters, Nature Communications (2023). DOI: 10.1038/s41467-023-39010-6
Journal information: Nature Communications
Provided by Brookhaven National Laboratory
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form. For general feedback, use the public comments section below (please adhere to guidelines).
Please select the most appropriate category to facilitate processing of your request
Optional (only if you want to be contacted back)
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
We keep our content available to everyone. Consider supporting Science X’s mission by getting a premium account.
This article has been reviewed according to Science X’s editorial process and policies. Editors have highlighted the following attributes while ensuring the content’s credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
48 shares
Cryo-EM study shows zinc transporter has built-in self-regulating sensor
Note:
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient’s address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Tech Xplore in any form.
About
Phys.org™ is a leading web-based science, research and technology news service which covers a full range of topics.
Phys.org is a part of Science X network. With a global reach of over 10 million monthly readers and featuring dedicated websites for science (Phys.org), technology (Tech Xplore) and medical research (Medical Xpress), the Science X network is one of the largest online communities for science-minded people.
Science X Account
Forgot Password?
Not a member? Sign up.
Identify the news topics you want to see and prioritize an order.
Science X Daily and the Weekly Email Newsletter are free features that allow you to receive your favorite sci-tech news updates in your email inbox
© Phys.org 2003 – 2023 powered by Science X Network
Newsletter
Science X Daily and the Weekly Email Newsletters are free features that allow you to receive your favourite sci-tech news updates.
Please, allow us to send you push notifications with new Alerts.
Your Privacy
This site uses cookies to assist with navigation, analyse your use of our services, collect data for ads personalisation and provide content from third parties.
By using our site, you acknowledge that you have read and understand our Privacy Policy and Terms of Use.